When hospitals implement a new high-level disinfection (HLD) process, the conversation usually focuses on speed, workflow, and compliance.
But Infection Prevention teams know the truth:
No HLD process is stronger than the steps that come before it.
Across chemical systems, washer-disinfectors, thermal methods, and UV-C, one universal rule remains unchanged:
Effective pre-cleaning is essential.
This isn’t just best practice. It’s explicitly reinforced by European infection prevention guidance. For example, ESGE guidance states that “a thorough cleaning of the endoscope is a prerequisite for proper disinfection.”
In other words, the pre-clean step isn’t optional, it’s foundational.
And when hospitals evaluate UV-C high-level disinfection for ENT, Cardiology or Women's Health workflows, one of the most important implementation questions often isn’t about the UV-C cycle itself.
It’s about pre-cleaning confidence.
The Infection Prevention Question That Always Comes First
When Vijf Meren Kliniek / Spaarne Gasthuis (Haarlem & Hoofddorp, Netherlands) evaluated the UV Smart D60 to upgrade their channel-less ENT endoscope high-level disinfection process, their Infection Prevention team raised a familiar concern:
How can we be sure pre-cleaning is being completed consistently and effectively?
That question isn’t skepticism, it’s responsible quality leadership.
Because pre-cleaning is the step that:
- removes visible contamination and organic soil
- reduces variability between users and shifts
- supports consistent disinfection outcomes
- improves audit readiness and traceable quality assurance
- strengthens clinician trust in the workflow
Why Pre-Cleaning Matters, No Matter Which HLD Method You Use
Hospitals don’t just need disinfection.
They need disinfection they can trust on every shift, with every staff member, every day.
That’s why pre-cleaning matters.
Pre-cleaning is not a “nice-to-have,” and it’s not specific to UV-C. It applies to every pathway. As professional reprocessing standards make clear:
Cleaning must precede disinfection or sterilization.
And because consistency matters, many standards emphasize that cleaning is part of the full processing chain, not a side step.
One globally recognized standard used widely in healthcare device processing is ISO 17664-1:2021, which specifies manufacturer-provided processing instructions for critical and semi-critical devices.
The takeaway is simple:
If pre-cleaning is inconsistent, any HLD method becomes less reliable, regardless of whether it’s chemical, thermal, washer-disinfection, or UV-C.
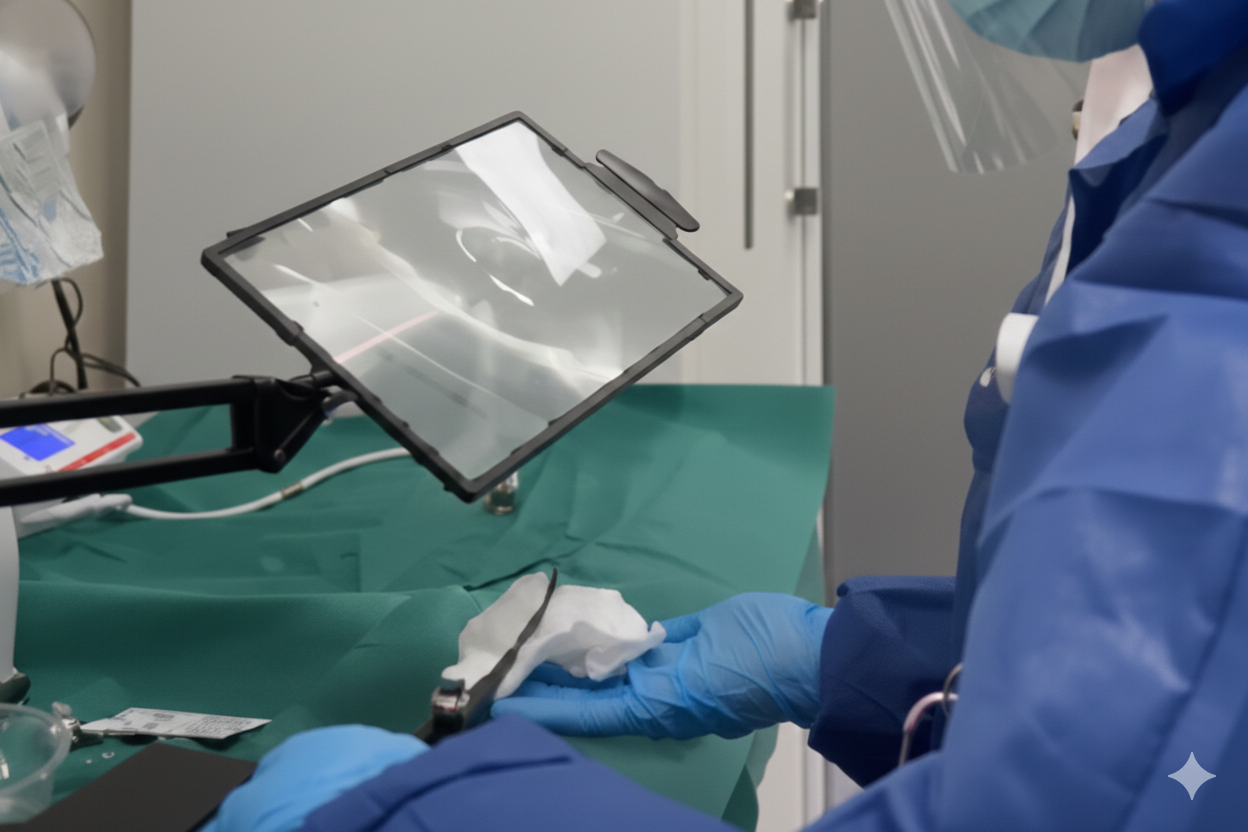
A Simple, Low-Cost Solution: The “Magnifying Glass Station”
To strengthen confidence across stakeholders without adding complexity, the hospital introduced a practical quality assurance step:
They placed a magnifying glass at the pre-cleaning station.
This allowed staff to complete standard pre-cleaning while adding a fast visual confirmation step:
- no visible debris remains
- no visible contamination remains
…before every high-level disinfection cycle.
This “magnifying glass station” works because it transforms an invisible concern into an observable safeguard.
Instead of relying on assumptions (“I think it’s clean”), the workflow becomes:
We checked.
Why This Works: It Strengthens Quality Assurance Without Creating Friction
In real clinical environments, adoption depends on more than written policies. It depends on what’s practical and repeatable.
This implementation tip worked because it supports what Infection Prevention teams want most:
1) It increases consistency
Visual inspection reduces variability between staff and shifts.
2) It strengthens Infection Prevention confidence
IP teams gain reassurance that cleaning isn’t assumed, it’s verified.
3) It supports audit readiness
Audits demand proof. Visible inspection reinforces standardization and consistency.
4) It doesn’t disrupt workflow
This isn’t a new protocol, it strengthens an existing one.
What This Improvement Did NOT Do (And Why That Matters)
One of the most important outcomes of this story is what it did not change.
This quality reassurance step:
- did not alter protocols that were already in place
- did not add burden to the clinical staff
- did not improve our 6-log reduction, this is already achieved with the devices
Instead, it demonstrated proactive commitment to quality assurance, while keeping the disinfection workflow validated and consistent.
Evidence-Based UV-C Disinfection: Validation Still Matters
Pre-cleaning is essential, but hospitals also need confidence that the HLD method itself delivers measurable, repeatable effectiveness.
That’s why UV Smart emphasizes evidence-backed performance.
For example, in an independent evaluation conducted at Technological University Dublin (TU Dublin), UV Smart reported performance outcomes supporting HLD expectations, including:
- ≥6-log reduction (or better)
- evaluation aligned with EN 14885 expectations
- testing performed under two real-world conditions: with standard pre-cleaning and without pre-cleaning
You can request the full report here:
Request TU Dublin Full Study
This type of testing matters because it supports what stakeholders care about:
- measurable disinfection performance
- repeatability under workflow pressure
- confidence in clinical reliability
If your team is in the early research phase, you can also reference this overview guide which highlights validated performance and compatibility questions hospitals ask first:
UV Smart Device Compatibility: 5 Questions Hospitals Ask
Key Takeaway: Confidence Drives Adoption
The experience of Vijf Meren Kliniek / Spaarne Gasthuis highlights something Infection Prevention and clinical leaders already know:
Implementation success is not only about performance, it’s about confidence.
Sometimes the best improvement isn’t adding complexity or rewriting policy.
Sometimes it’s a small, practical tool that strengthens execution at the point of care without slowing teams down.
A magnifying glass at the pre-cleaning station is a simple implementation improvement, but it reinforces a culture of inspection, standardization, and workflow reliability.
And in high-level disinfection, that matters.
Want to Upgrade HLD With Confidence?
If your hospital is evaluating UV-C high-level disinfection methods, UV Smart can support implementation planning, Infection Prevention stakeholder alignment, and workflow design.
Next step options:
- Request a UV Smart workflow consultation
- Speak with a real users of UV Smart devices
- Get adoption and implementation resources
FAQ: Pre-cleaning + Validated HLD Effectiveness
Is pre-cleaning required before high-level disinfection (HLD)?
Yes. Pre-cleaning (meticulous cleaning) is required before any high-level disinfection or sterilization method regardless of the technology used.
CDC explicitly states: “Meticulous cleaning must precede any high-level disinfection or sterilization process.”
Does this apply to all HLD methods (chemicals, washer-disinfectors, thermal, UV-C)?
Yes. The requirement is universal. Organic debris and visible contamination can interfere with disinfection effectiveness, which is why cleaning and inspection must occur before any HLD method can be trusted.
What happens if pre-cleaning is skipped or done poorly?
When cleaning is inconsistent or incomplete, the entire disinfection process becomes less reliable, creating preventable risk and increasing the chance of process failure. However, you can request how UV Smart achieves necessary log reduction standards.
UV Smart supports evidence-based adoption through validated performance evaluations, including independent testing. These tests are designed to reflect real-world conditions and worst case scenarios.
Does UV Smart rely on pre-cleaning?
Yes. Like all high-level disinfection pathways, UV Smart workflows are built on following regulations for HLD that say all HLD reprocessing methods must have a pre-cleaning step. UV-C disinfection is designed to deliver a standardized, measurable disinfection outcome once the device is properly prepared.



.jpeg)



.jpg)
